When researchers at Northwestern University started researching sodium-ion batteries, they had one uniform goal – find commonly abundant materials for a cheap alternative to lithium ion batteries. The depletion of lithium sources makes it essential to find alternative sources of battery power – soon.
By Puja Bhattacharjee
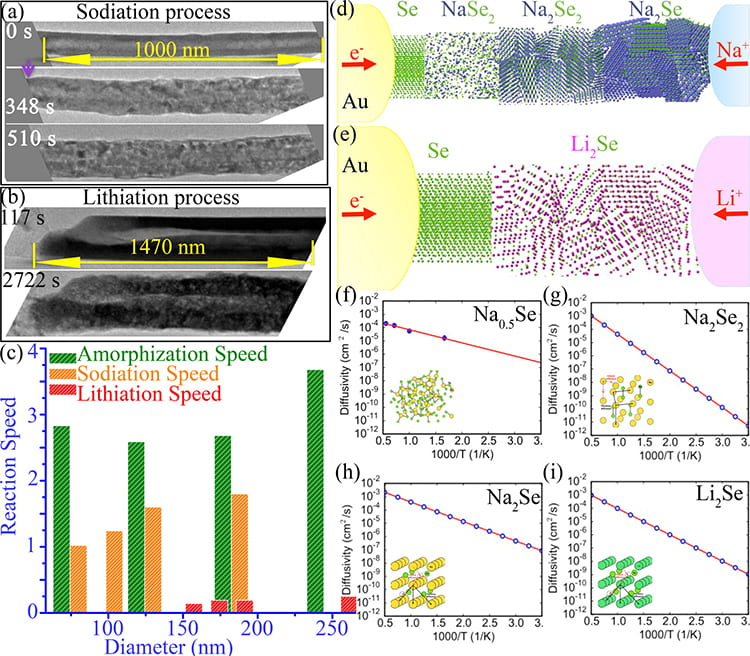
Researchers in the Department of Material Science and Engineering at Northwestern University are developing and testing a sodium-selenium battery. They found that sodium reacts faster and charges rapidly without losing capacity. And sodium is highly available in earth in the form of sea salts.
Lithium batteries developed in the 1980s changed the way technology works in the 21st century. Be it the laptop, cell phone or any other portable electronic device, all of them run on lithium ion batteries. Moreover, lithium batteries are light, safe, have high capacity i.e. they can store more energy and are environmentally friendly compared to previous batteries like lead acid batteries.
What is Lithium?
Lithium is a highly reactive silver-white metal which occupies the third position in the periodic table. Also, known as ‘white petroleum,’ it is abundantly found in Argentina, Bolivia and Chile, collectively known as South America’s ‘lithium triangle’. The cost of doing business in these countries is often quite high, leading to higher costs for the resulting lithium.
Decades later, with the progress in technology, there is a need for more capacity and service life in batteries. “Lithium ion batteries are 25 years old. They do not have as much energy or power density as some of the new ideas that are coming in the market,” says Prof. Vinayak Dravid, Director of the SHyNE Resource.
Moreover, lithium sources are depleting. In 2016, Credit Suisse estimated that demand for lithium could outstrip supply in 2020 by 25 percent. That’s how scientists started looking for alternative sources of battery power.
They focused on sodium which is right below lithium in the alkali metal group of the periodic table. The other metals of the group have unstable reactivity which makes them unsafe to handle.
Sulphur is the cathode material for the sodium ion battery. The sodium-sulphur combination proved to be a disadvantage because the two elements react with each other only at very high temperatures, of no use for every day electronic items which are maintained at room temperature.
Researchers then selected selenium as the cathode material for a sodium ion battery. Selenium can react with sodium at room temperature.
How and what did the researchers study?
Researchers synthesized selenium nanotubes and used one as the cathode material for a nano battery. The nano battery was placed inside the transmission electron microscope (TEM), located at NUANCE, one of the centers of the SHyNE Resource at Northwestern University. They observed the reaction between selenium and sodium during the process of charging the battery. By studying the reaction, the researchers found that the battery had high chargeability. This observation made selenium a promising cathode material.
The next challenge is to substitute selenium with something much cheaper. “The idea behind this research was to demonstrate [feasibility] and that’s how typically science innovation works,” adds Dravid. Science is about creativity without too much concern over cost, he says. The team’s next step is to take the scientific breakthrough and tailor it in a way that it would yield a cost-effective high functioning battery.
“We are now looking for a material which has intermediate phases (with high) conductivity. We will explore other materials which can function just like selenium to reduce the cost,” says Dravid.
Since selenium is expensive, alternate anode materials must be explored. One method being explored is to “dope” sulphur with selenium, creating an alloy, says researcher Qianqian Li, a postdoctoral research associate, NUANCE Center.